This contrasts with the historical pattern in which testing was wholly or mostly confined to the medical laboratory which entailed sending off specimens away from the point of care and then waiting hours or days to learn the.
Point of care testing devices pdf.
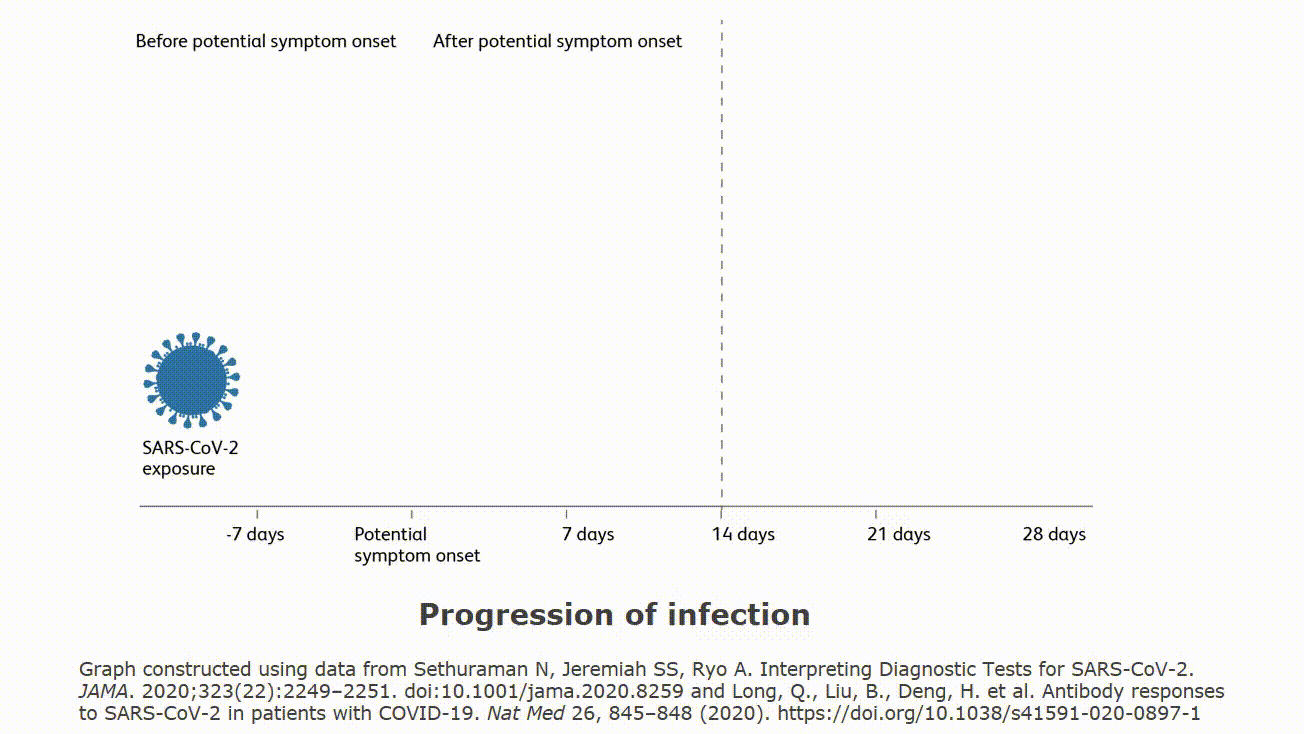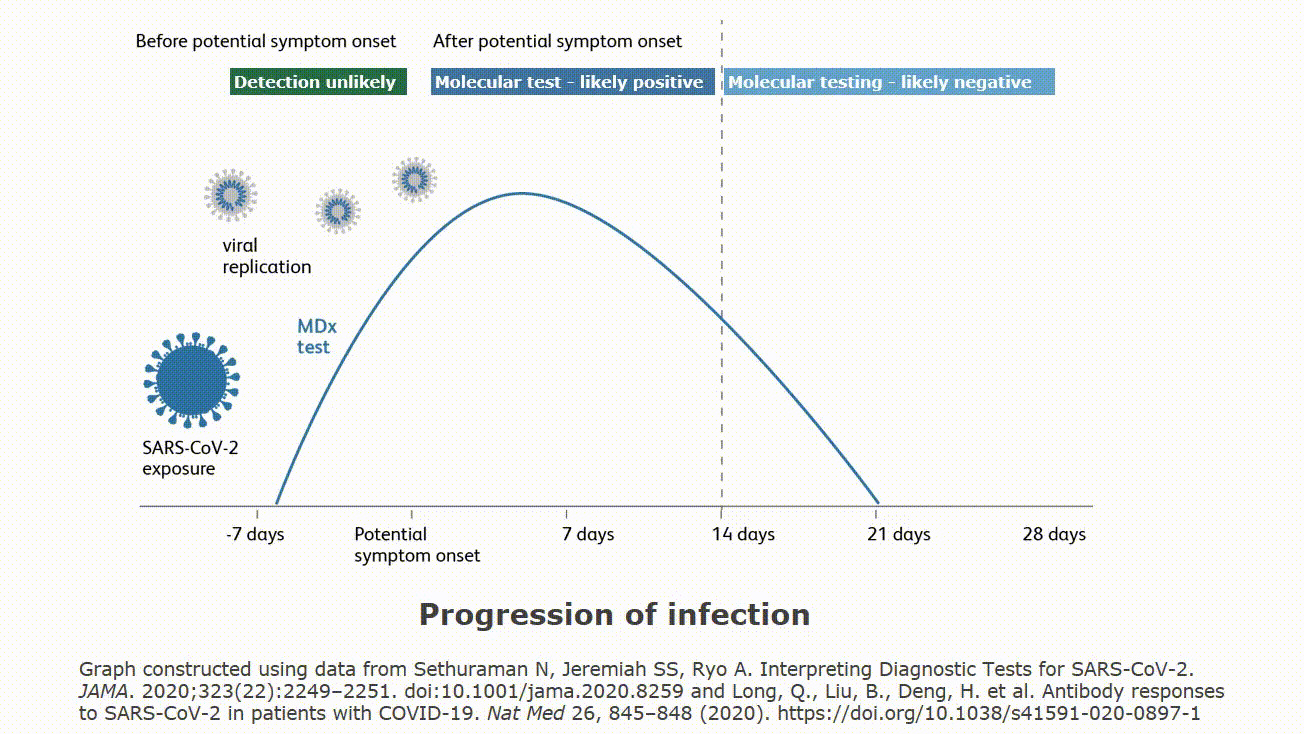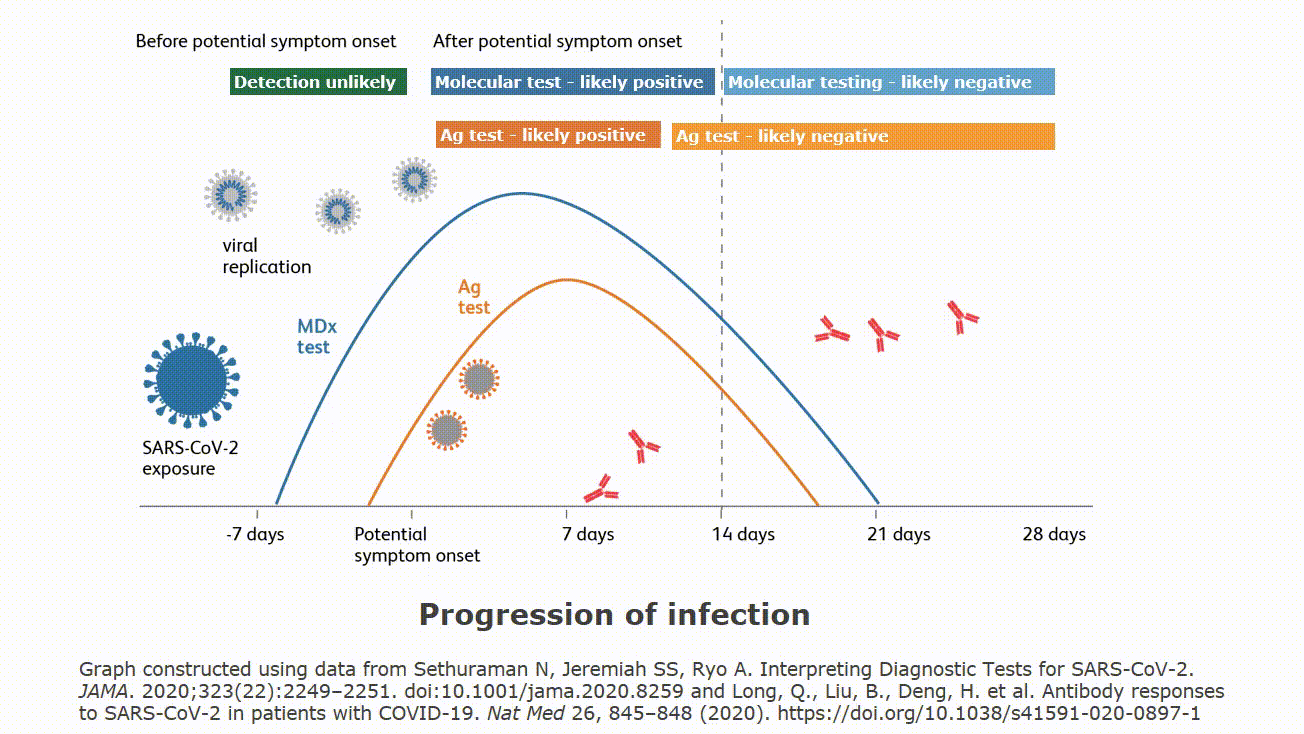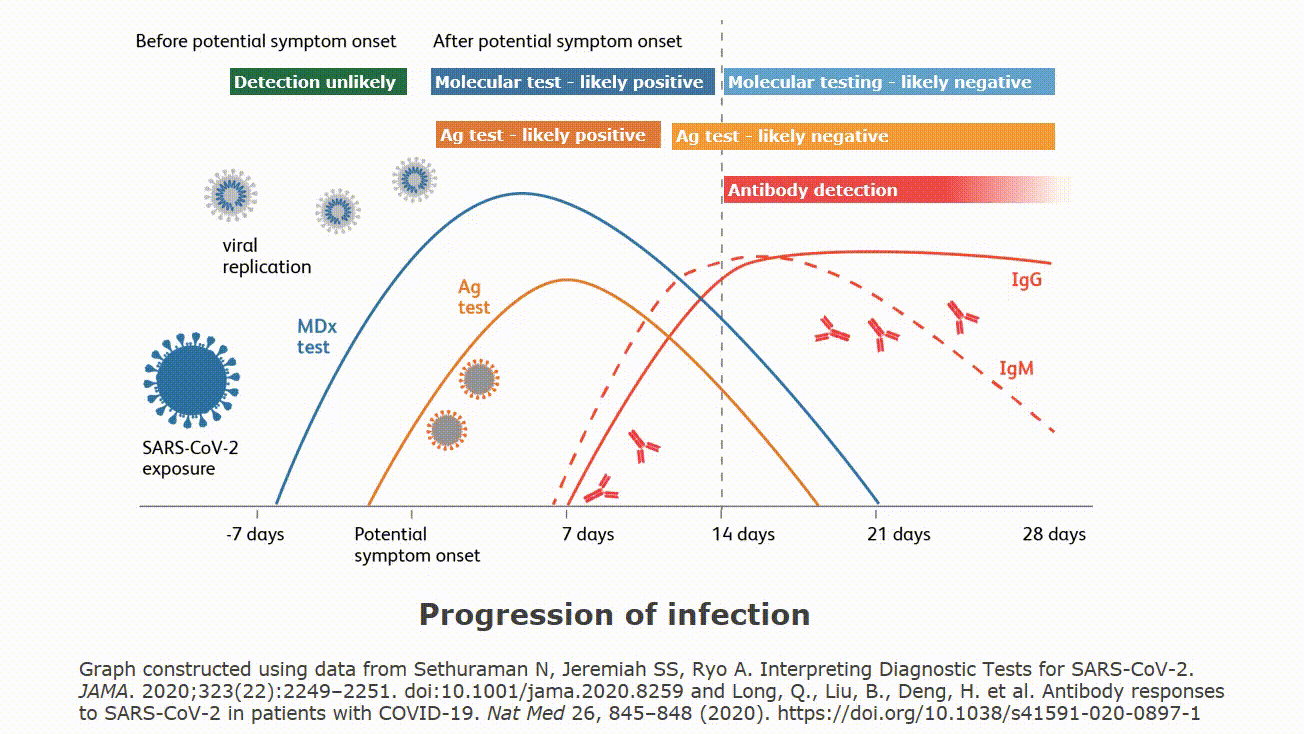
Testing is called point of care testing poct and is defined as testing at the point where patient care is given wherever that is located.
Point of care testing requires microfluidic precision.
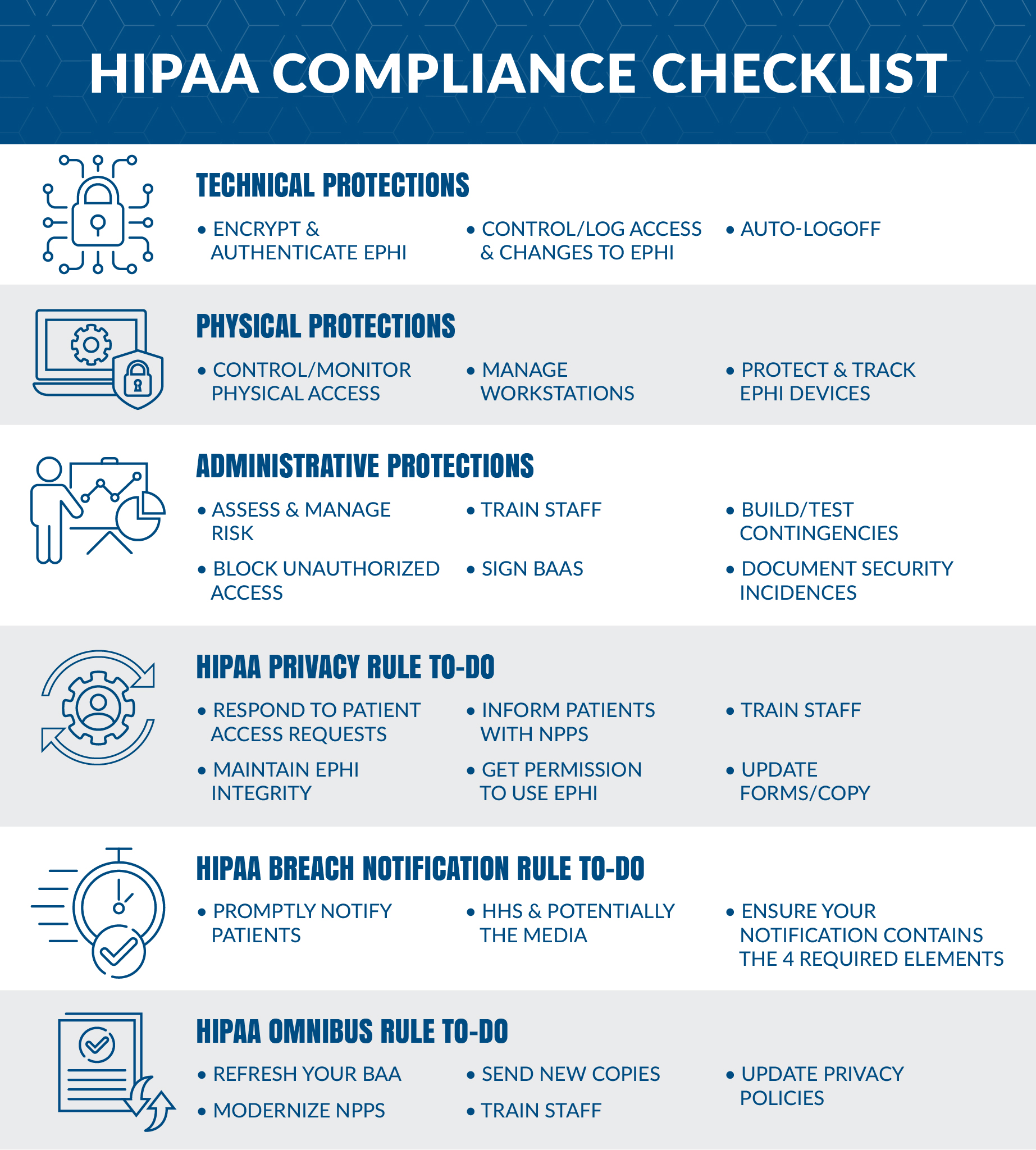
Policy and procedures for the management of point of care testing poct devices.
The aim of this policy is to ensure that poct evices are subject to d.
Point of care testing poct or bedside testing is defined as medical diagnostic testing at or near the point of care that is at the time and place of patient care.
This expansion is driven by an increasingly diverse array of advanced medical diagnostic equipment that can be used at or near the point.
1 2 poct devices are used widely uhl for diagnosis monitoring and across treatment.
Nursing facilities will receive one of two testing devices.
Point of care antigen test devices on july 14 centers for medicare and medicaid cms announced an initiative to distribute rapid point of care poc antigen covid 19 testing devices to nursing homes across the country.
Point of care testing often starts without knowing if the testing is appropriate for the setting.
With this move outside the laboratory walls some problems occur that were not problems within the laboratory.















.jpg)





