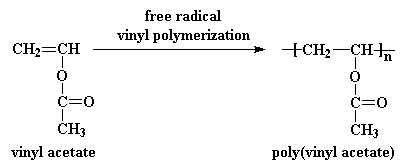
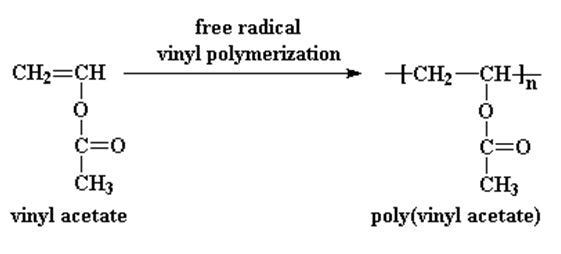
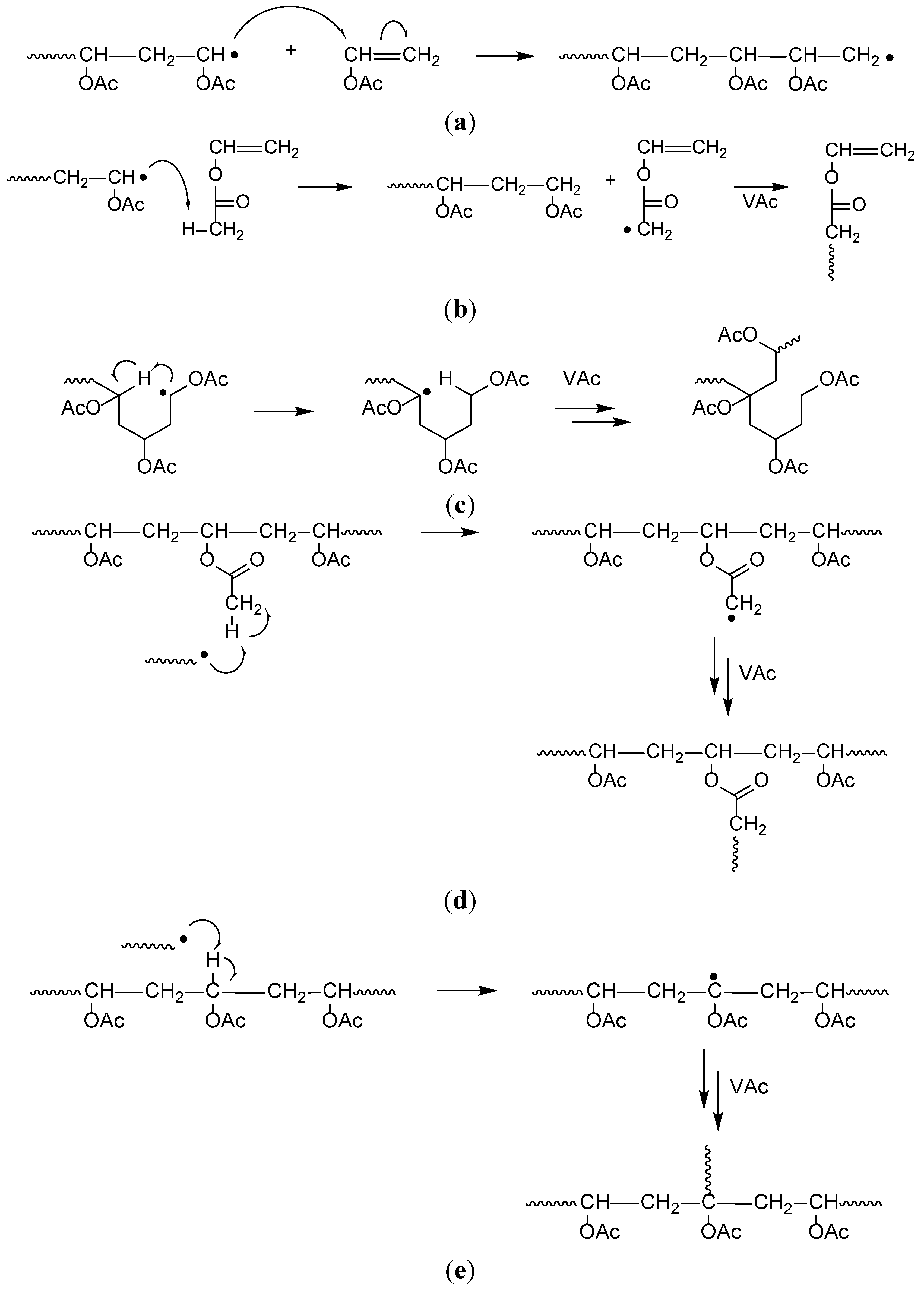
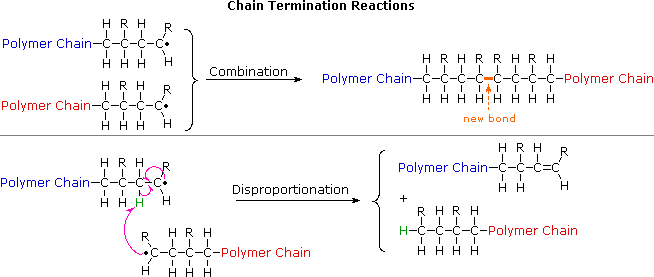
The polymerization of vinyl acetate is probably the second most frequent cause of runaway reaction accidents in the chemical industry after the phenol formaldehyde runaway reaction.
Polymerization vinyl acetate.
Learn more about polyvinyl acetate in this article.
The controlled polymerization was photoresponsive.
Thus the type of emulsion polymerization process batch semi continuous or.
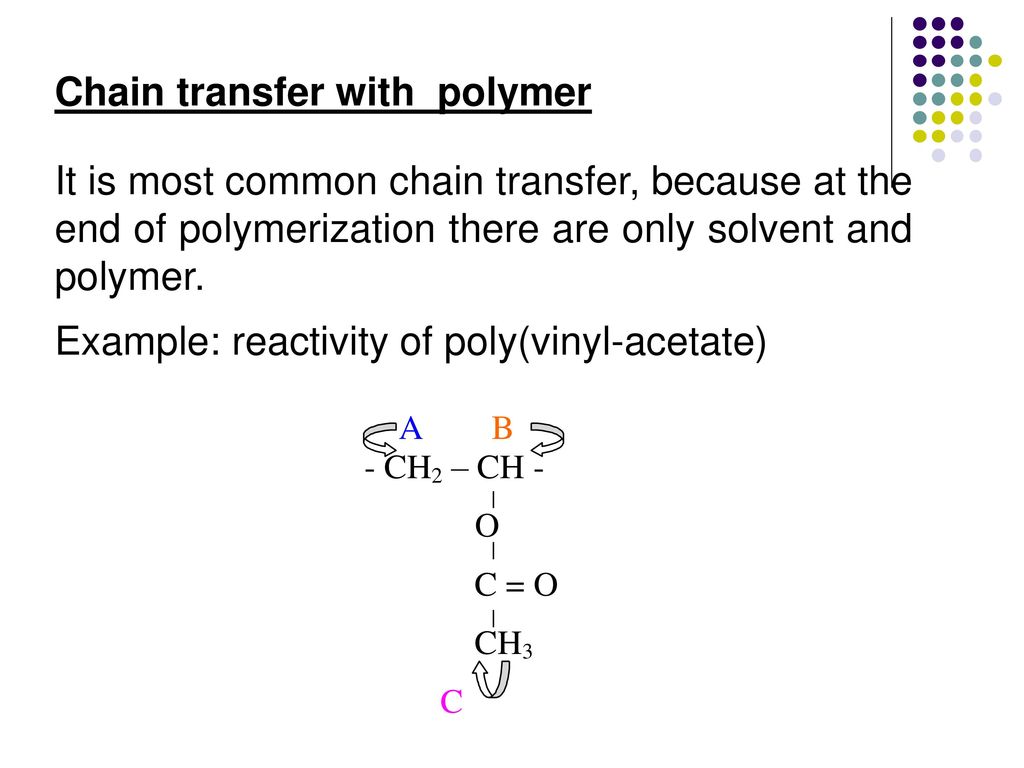
Vinyl acetate has high water solubility a high monomer polymer swelling ratio and a high chain transfer constant.
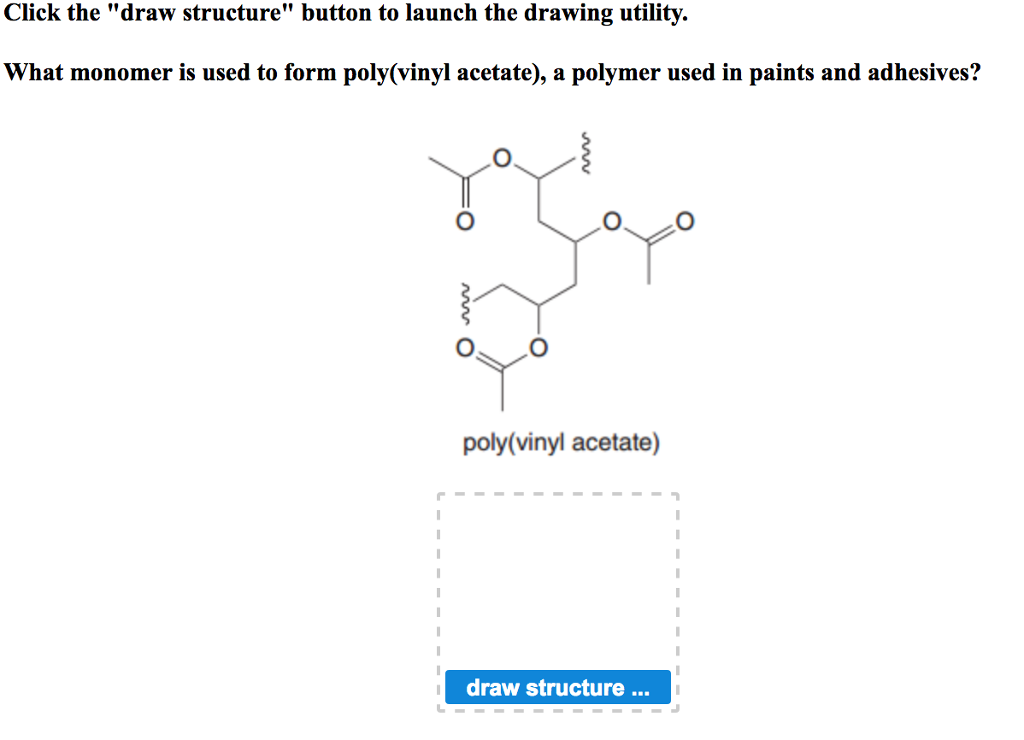
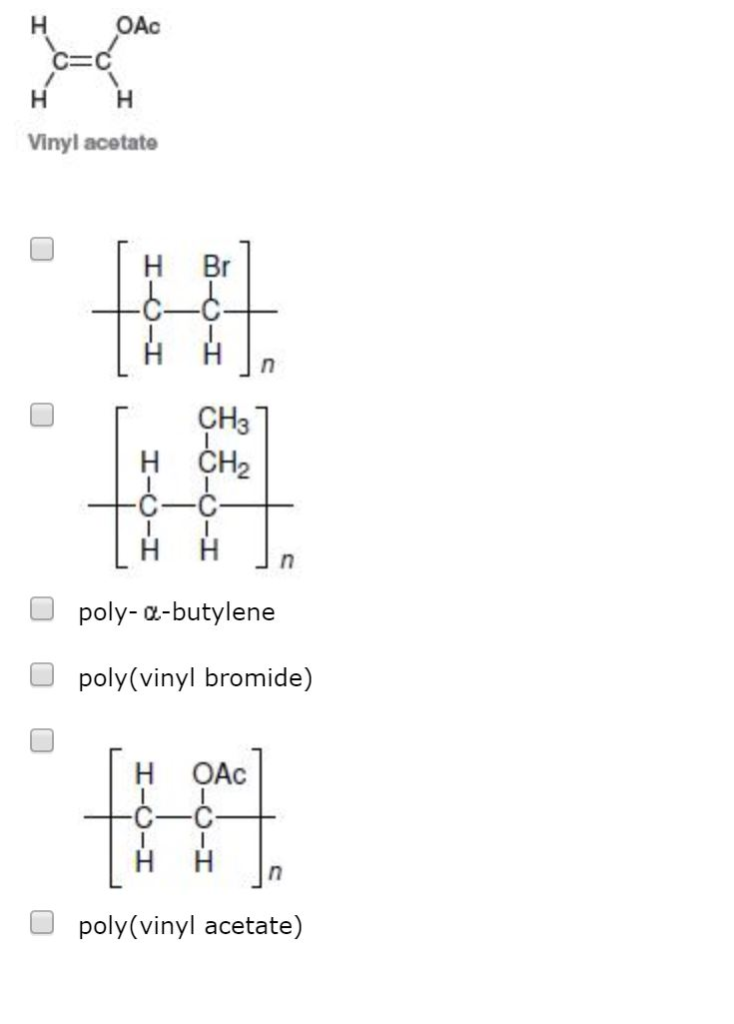
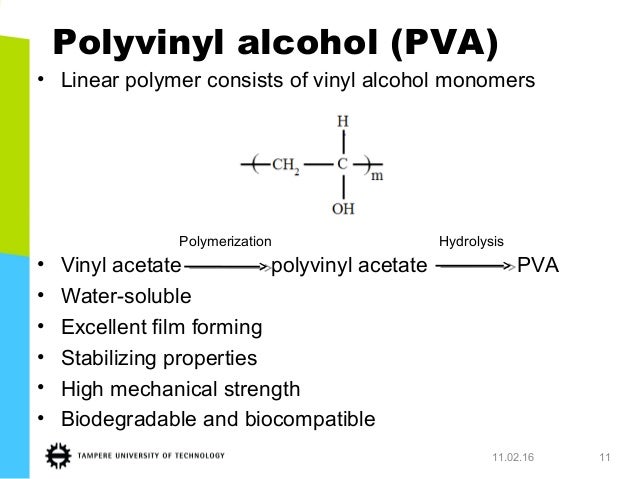
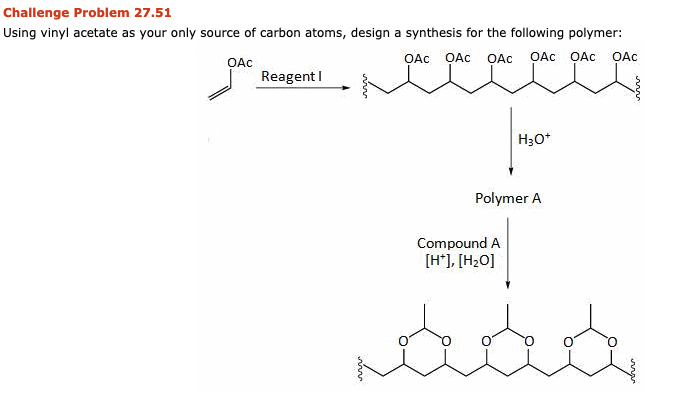
Polyvinyl acetate is prepared by the polymerization of vinyl acetate monomer free radical vinyl polymerization of the monomer vinyl acetate.
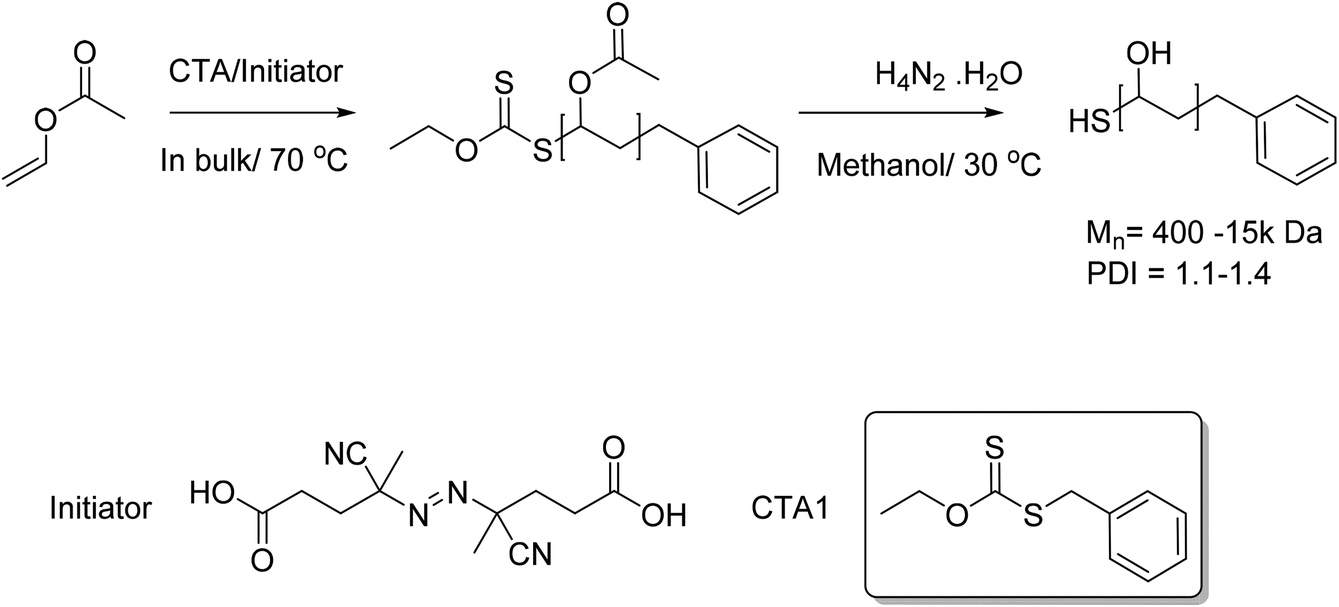
The photoinduced polymerization of vinyl acetate vac proceeded very fast even at 40 c with a catalytic amount of mn 2 co 10 0 025 mol of vac or 5 0 mol of r i and completed within a few hours to give the polymers with controlled molecular weights up to 10 5.
It is also used in adhesives.
In the present report the madix polymerization of vinyl acetate in bulk or solution polymerization dichloromethane dcm or ethyl acetate was investigated in order to optimize monomer conversion and achieve molecular weight control.
Applications edit as an emulsion in water pvac emulsions are used as adhesives for porous materials particularly for wood paper and cloth and as a consolidant for porous building stone in.
Polyvinyl acetate adhesives pvac polyvinyl acetate latex known as white latex is made of vinyl acetate monomer water dispersing agent polymerization starter and other auxiliary materials mixed together through emulsion polymerization.
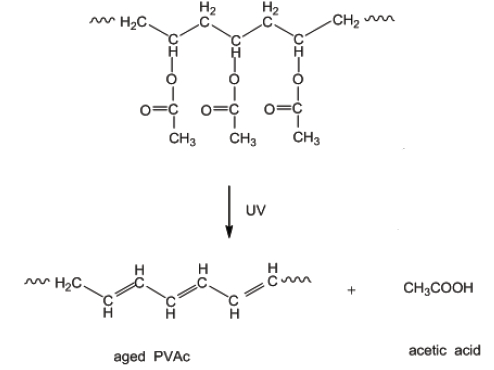
Vinyl acetate may undergo spontaneous exothermic polymerization on exposure to light.
Reacts with hydrogen peroxide to form explosive peracetic acid.
Since vinyl alcohol is highly unstable with respect to acetaldehyde the preparation of vinyl acetate is more complex than the synthesis of other acetate esters.
In its most important application polyvinyl acetate serves as the film forming ingredient in water based latex paints.
All polymerization reactions were performed using a facile approach using 15 ml glass pressure tubes with.
The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst.
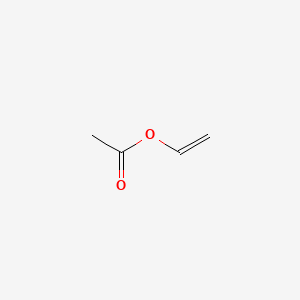
Vinyl acetate is the acetate ester of vinyl alcohol.
Vinyl acetate c4h6o2 cid 7904 structure chemical names physical and chemical properties classification patents literature biological activities safety.
Reacts with air or water to produces peroxides that initiate explosively violent polymerization.
It is a nonstructural adhesive with low price convenient usage and wide application range.
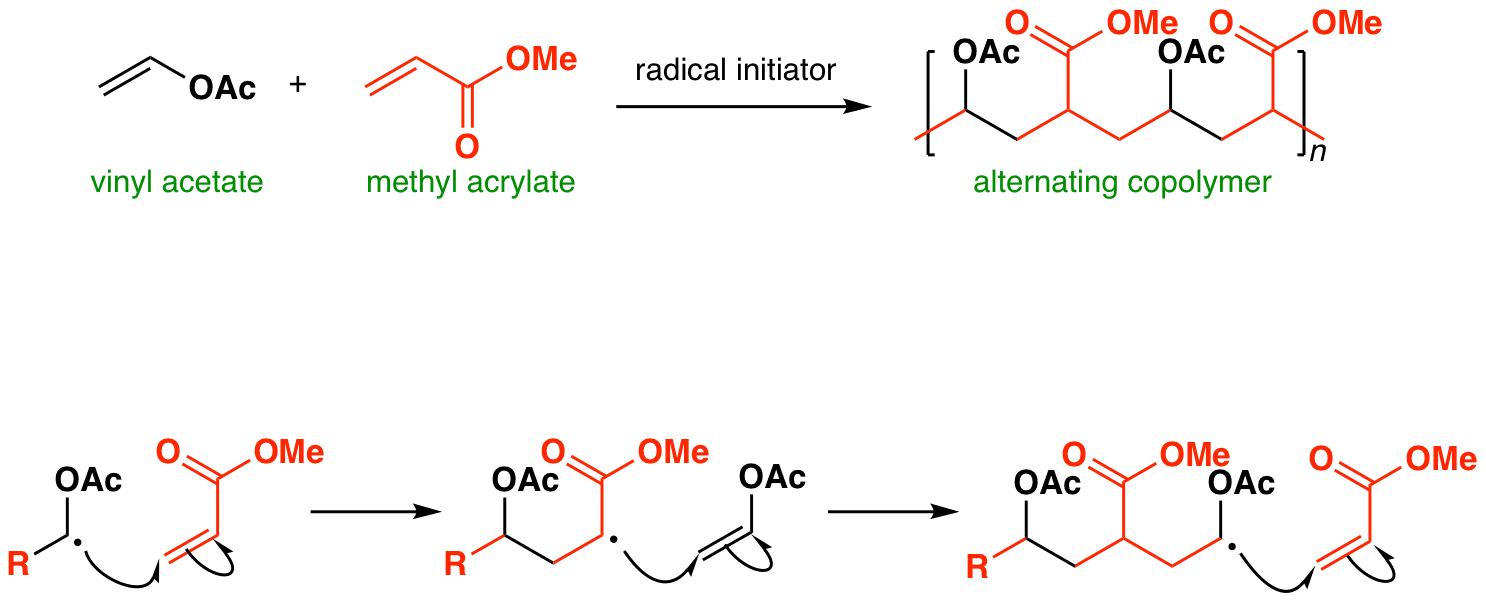
The emulsion polymerization of vinyl acetate possesses the rather typical properties in comparison the emulsion polymerizations of the comonomers.