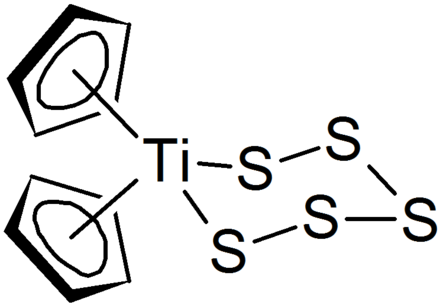Polysulfide rubber was discovered in 1926 by an american chemist joseph cecil patrick while he was attempting to obtain ethylene glycol for use as an antifreeze.
Preparation of polysulfide rubber.
The phase separation is brought about by using as a modifier a liquid polysulfide containing a high proportion of.
The invention relates to the field of coatings based on polysulfide rubber used in the technique of corrosion protection of metals.
Model preparation is minimal.
Polysulfide impression material was the first non aqueous elastomeric rubber impression material developed for dentistry.
A certain amount of cox was then added to the above molten liquid while stirring and heating were continued to ensure efficient mixing and reactions.
Once cured polysulfide molds are good for casting wax lost wax process and gypsum plasters.
A polysulfide modified epoxy resin system in which upon curing the polysulfide rubber phase separates into discrete particles typically 1 to 5 μm in size to enhance physical properties such as peel strength without any significant reduction in glass transition temperature.
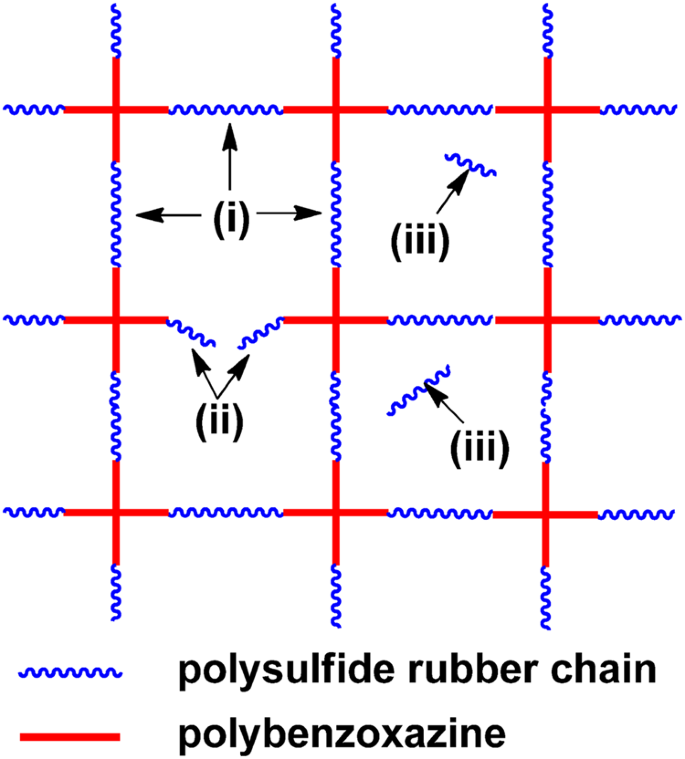
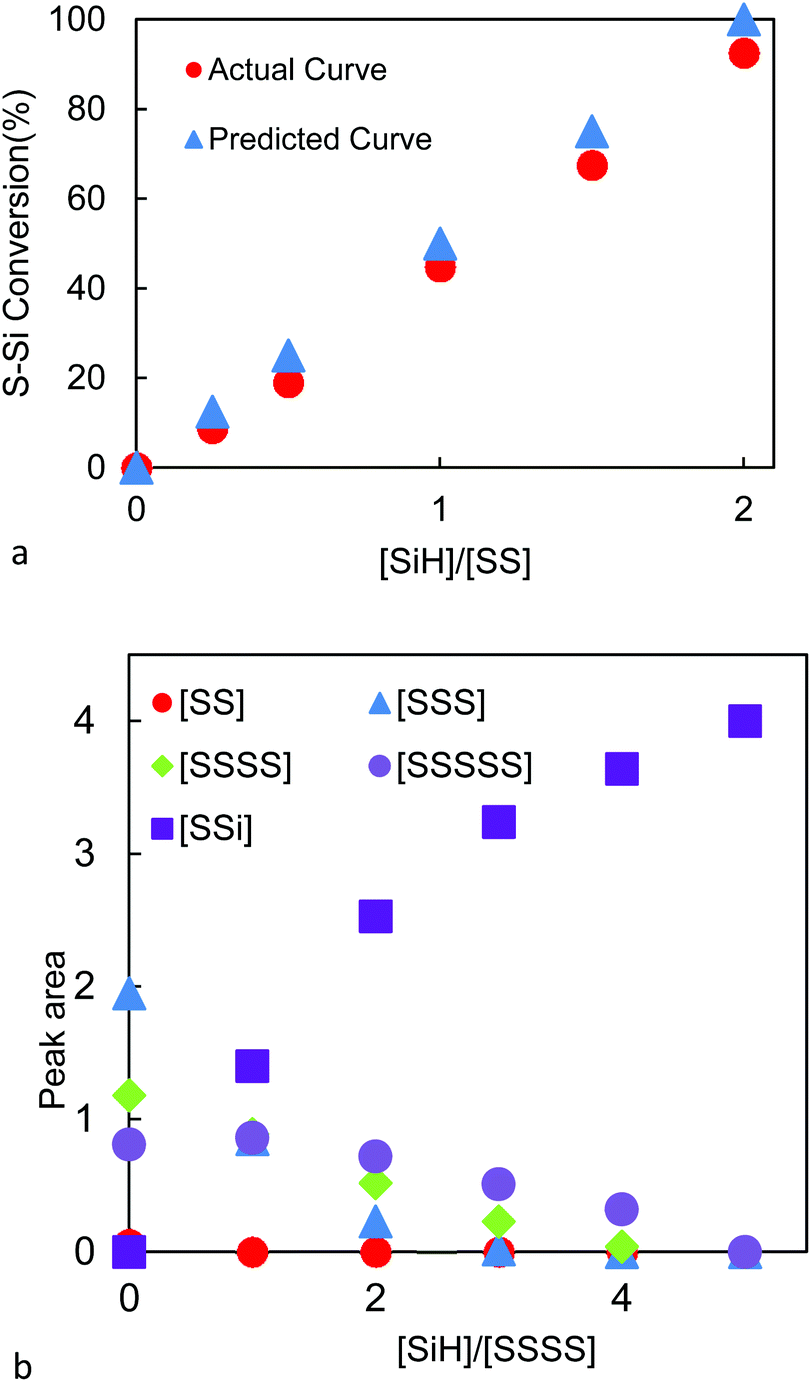
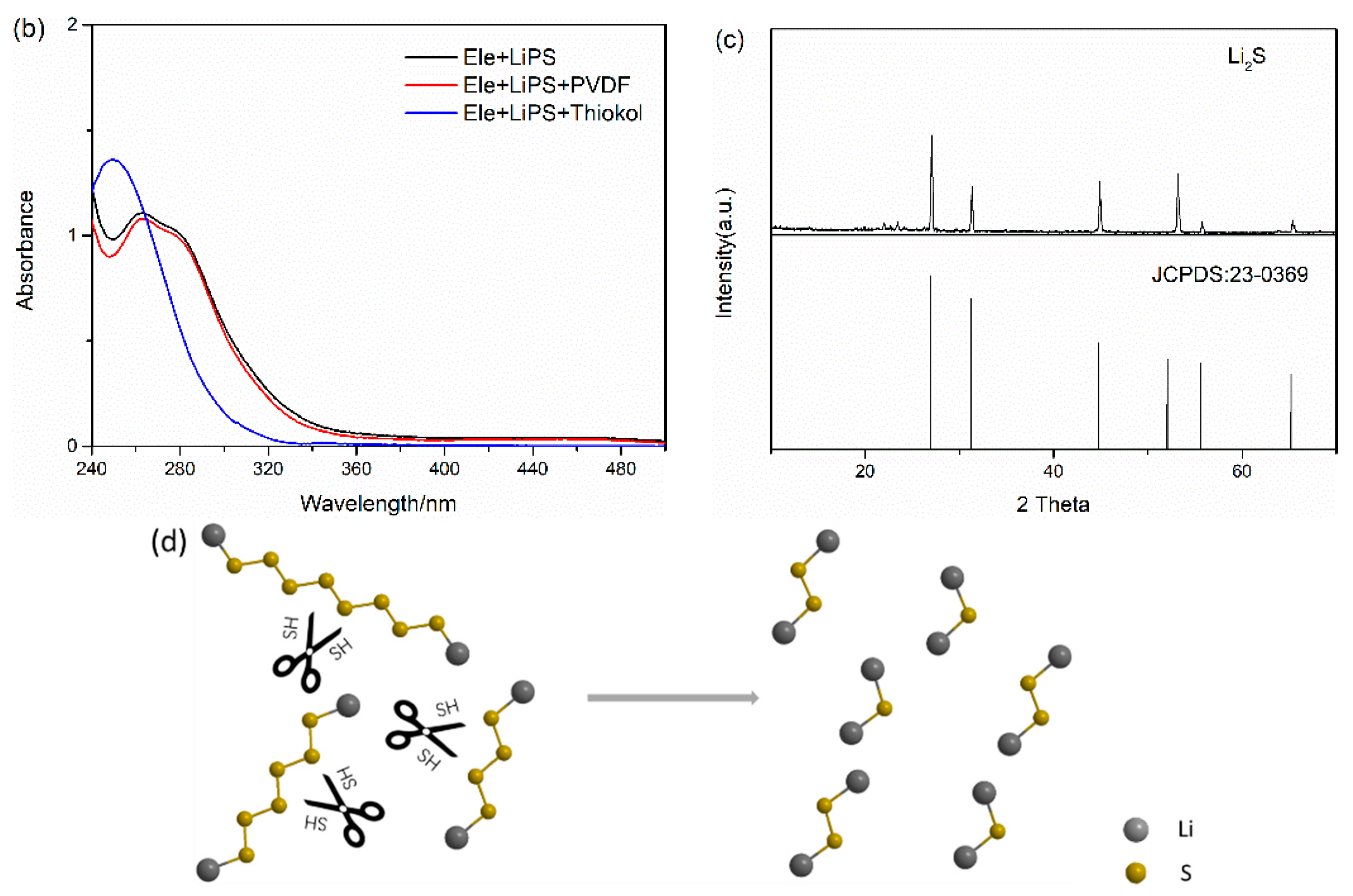
In this work a benzoxazine bridged by a flexible polysulfide rubber chain blp was conveniently prepared through the ring opening addition of thiol capped liquid polysulfide rubber lp 3 and.
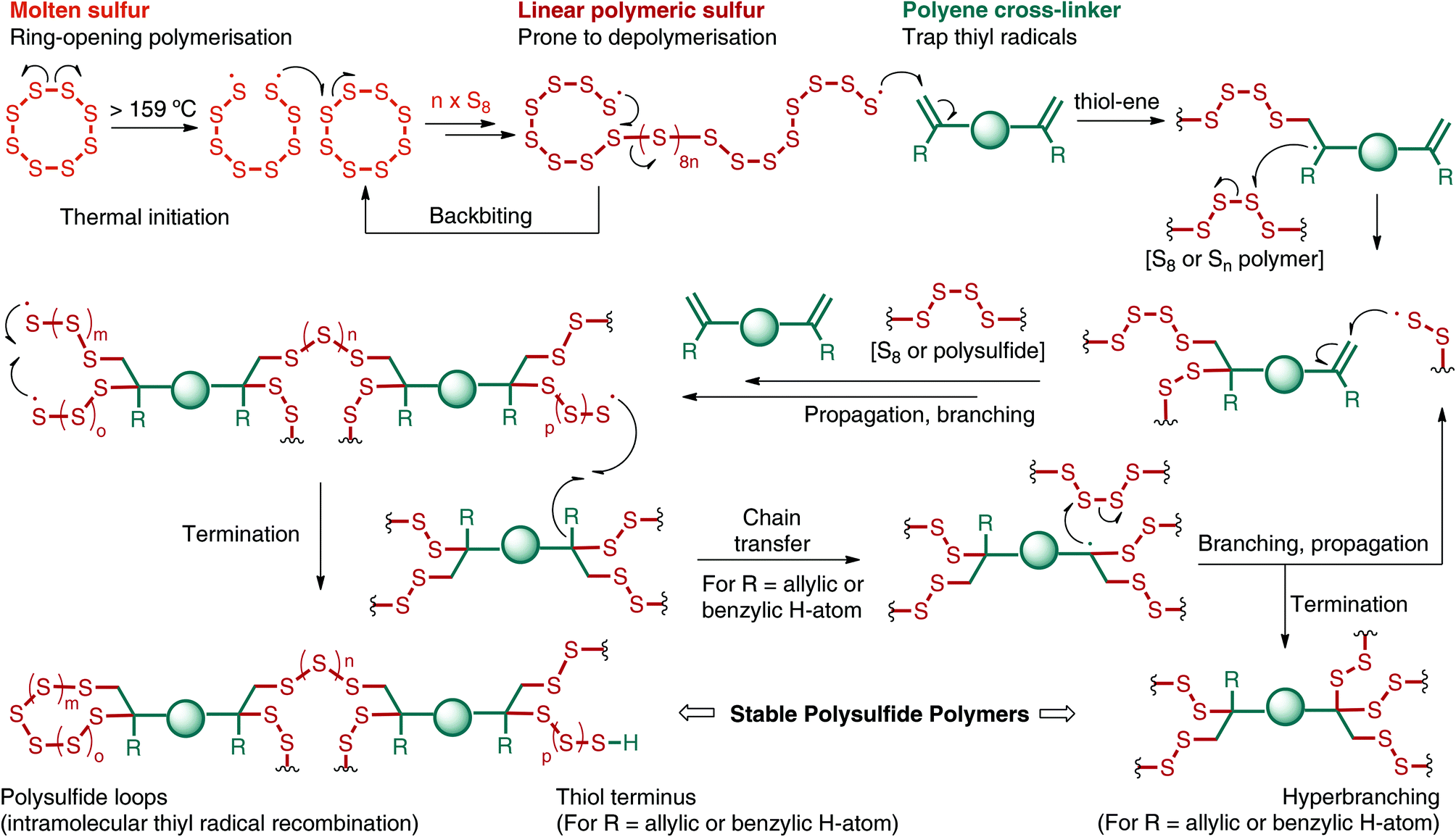
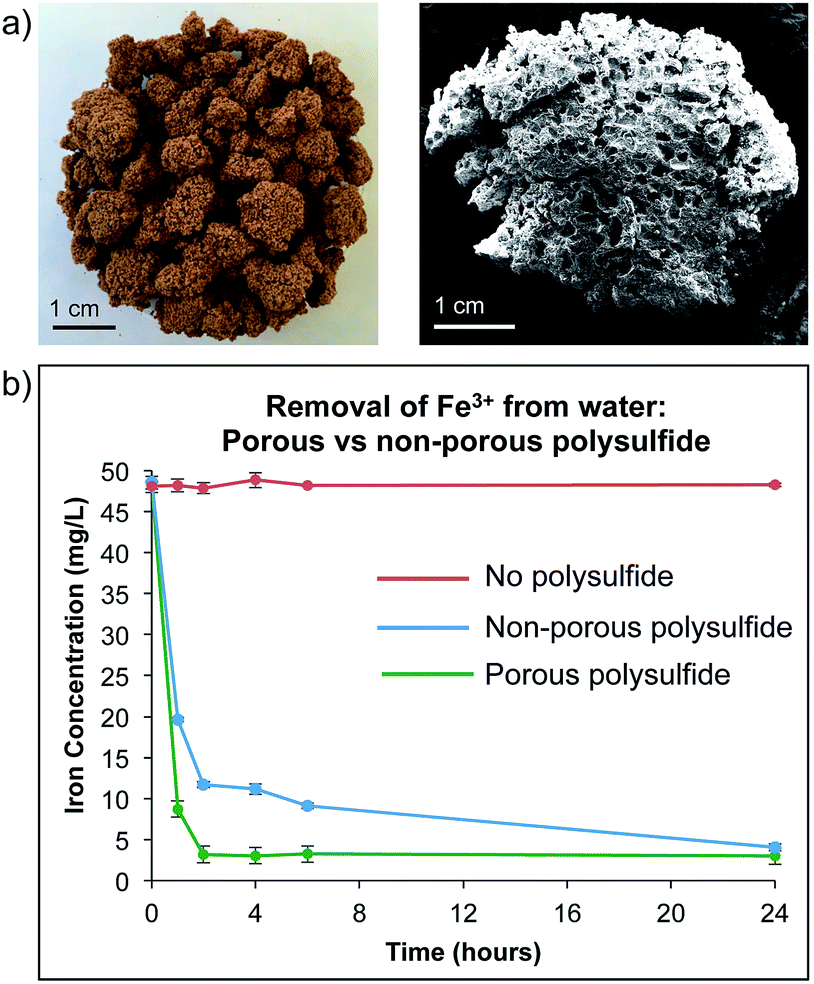
Preparation of polysulfide derived polymers about 10 0 g of elemental sulfur powder s was added into a 100 ml vial equipped with a magnetic stir bar and then melted while stirring at 150 c.
Other articles where polysulfide rubber is discussed.
A variety of thiokols are recognized.
For preparation for transition cured adhesive.
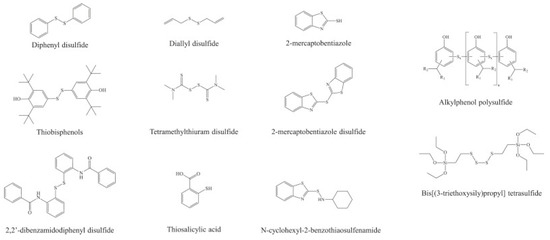
Disadvantages the most common polysulfide rubbers.
The distinction between the polymers first commercialized by the thiokol corporation and subsequent polysulfide materials is often unclear.
The elastomer was commercialized under the trade name thiokol after the greek theion brimstone sulfur and kommi.
Often polysulfide materials are called rubber or rubber base materials even though polyether and silicone materials are also rubber materials.
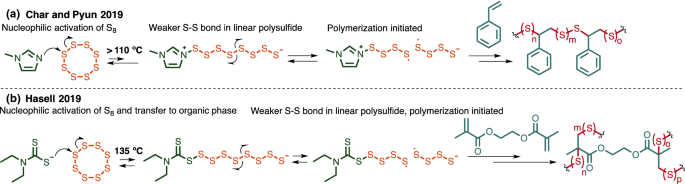
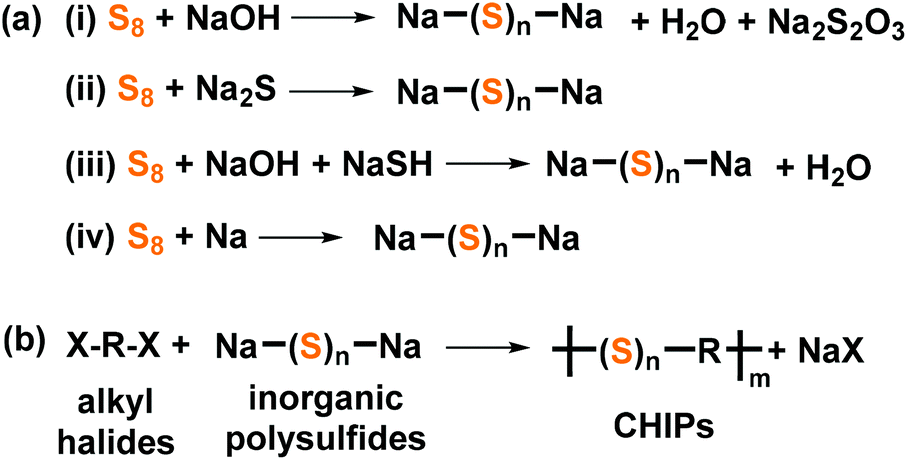
S8 2 naoh na2s8 at room conditions sulfur is normally in the form of s8 rings and chains.
The proposed solution can be used for example for long term protection equipment chemical plants power plants operated under variable effects of acid and alkali.
Mckeen in the effect of uv light and weather on plastics and elastomers fourth edition 2019.
The first step is the preparation of sodium polysulfide by the reaction of sulfur s8 with a strong base sodium hydroxide naoh.
The small scale preparation of thiokol rubber this preparation of thiokol rubber is a two step process.
Thiokol is a trade mark for various organic polysulfide polymers thiokol polymers are used as an elastomer in seals and sealants.